
Pools once closed at the first whisper of poliomyelitis. Parents eyed playgrounds with dread. Newspapers tallied cases like weather reports. In that world of iron lungs and summer quarantines, one research program reshaped the possible. It proved that careful method, civic cooperation, and steady ethics can beat back a terrifying disease and re-write the rhythm of everyday life. This is the story of a humane scientist, a mobilized public, and a vaccine that turned fear into prevention—and what the journey still teaches us about science, trust, and the way societies look after their children.
What follows is a fresh, fully original retelling designed for today’s readers. It blends biography, medical history, policy lessons, and the lived textures of the era—how trials were organized, how safety rules hardened after a crisis, and how schools, nurses, and volunteers became the invisible machinery of progress. It’s also an argument: the playbook that worked in the 1950s remains the best way to handle new microbial threats now—transparent trials, disciplined manufacturing, and straight talk with the public.
To understand why a vaccine could change the mood of a nation, start with the emotional climate it entered. Summer, once a season of relief, became a time of watchfulness. Families invented their own rituals: disinfecting doorknobs, skipping theatres, and scanning for news about the next town over. Stranger still was polio’s lopsided cruelty—most infections slipped by unnoticed, but a minority could permanently damage the spinal cord in children who had otherwise been thriving. There was no way to predict who would be spared and who would not.
Paradoxically, modern sanitation helped set the stage for those brutal seasons. As water systems improved, infants were less likely to meet the virus while still protected by maternal antibodies; first exposure arrived later in childhood, when the immune system faced the pathogen alone. Public health leaders knew a vaccine was the only durable solution. They just needed the tools—cell culture techniques that worked outside of living animals, clean trial designs that could withstand public scrutiny, and a manufacturing blueprint that made safety measurable rather than assumed.
The civic engine mattered as much as the science. Philanthropic organizing, most famously the March of Dimes, transformed private fear into a public project. Millions of small contributions funded lab benches, field nurses, and the massive logistics behind school-based trials. Parents did not merely consent; they participated, volunteered, and kept careful records. Out of that partnership grew a practical route from petri dish to pediatric clinic.
Why the Jonas Salk polio vaccine changed public health
Before widespread immunization, prevention meant avoidance—closing pools, cancelling picnics, reshuffling summers into sterile routines. Afterward, prevention meant reliable schedules, clinic visits, and a shrinking map of paralysis. The Jonas Salk polio vaccine reoriented the conversation from fatalism to logistics. It presented the immune system with a faithful portrait of the virus while denying the virus its power to replicate, and by doing so it shifted public life from emergency measures to ordinary planning. (For a plain-language historical overview, see this accessible CDC history of polio.)
Two features made the Jonas Salk polio vaccine transformative. First, its conservative, safety-first design reduced the possibility of vaccine-caused disease to vanishingly small odds when properly manufactured. Second, it could be produced in standardized lots, each tested for identity, potency, purity, and complete inactivation. That turned a scientific breakthrough into an industrial achievement: quality control and lot release were no longer backstage technicalities but the main stage for public safety.
Trust followed process. People were not asked to believe because a headline said so; they were shown schedules, data, and clear explanations of side effects. Community physicians and school nurses became translators of confidence, turning the Jonas Salk polio vaccine from a new technology into a familiar ritual—one more line on the family calendar, one more stop during well-child visits.
Inside the lab: making the Jonas Salk polio vaccine
Inactivated (killed-virus) vaccines ask for delicate chemistry. The virus must be neutralized so it cannot reproduce, yet the three-dimensional shapes of its outer proteins must remain intact so the immune system can learn them. That balance was achieved with formalin in carefully controlled conditions. Lots were sampled and tested repeatedly—before, during, and after inactivation—to ensure that every vial behaved like the models on which the trials were based. This discipline turned the Jonas Salk polio vaccine from a bench recipe into a repeatable product that could be trusted by clinicians who had never met the scientists who designed it.
Public communication mirrored that discipline. The language was measured, the benefits and risks spelled out without hedging, and the dosing schedules explained so parents could plan around them. By the time school gymnasiums hosted vaccination lines, the science was not merely published—it was intelligible. In that landscape, the Jonas Salk polio vaccine moved quickly from novelty to norm.
How the Jonas Salk polio vaccine was tested and trusted
Scale can create confidence, but only when paired with independence. The massive field studies enrolled enormous numbers of schoolchildren and deliberately separated the developer from the evaluator. Local health workers organized sign-ups and lines; an outside academic center adjudicated the outcomes. That structural modesty mattered: it meant that conclusions about the Jonas Salk polio vaccine did not depend on the inventor’s enthusiasm, but on the measurements of colleagues who had every incentive to be cautious.
The signal was clear—children who received the shots were strongly protected from paralytic disease. Communities not only saw statistics; they recognized the faces behind them: classmates, cousins, neighbors. Rather than demanding trust, the program earned it. When headlines celebrated these results, doctors’ offices filled, and the Jonas Salk polio vaccine earned its authority the right way: by working, and by proving it in full view.
Success arrived with a painful lesson. A manufacturing failure at one company released lots that were not fully inactivated, causing clusters of disease. The national program paused. Investigations were immediate and public. Standards were rebuilt—tighter in-process controls, deeper lot-release testing, more intrusive inspections. The institutional message was bracingly adult: safety is not a promise; it is a structure. That reconstruction restored confidence in the Jonas Salk polio vaccine and strengthened every biologic that followed.
For families seeking a patient-friendly primer on present-day schedules and safety, a concise, up-to-date overview is available from MedlinePlus (U.S. National Library of Medicine). It complements historical sources by focusing on practical questions—dosing, side effects, and the role of polio shots in routine pediatric care—while clinicians continue to rely on provider-facing guidance. Resources like this help keep the conversation steady around the Jonas Salk polio vaccine and its place in modern schedules.
After 1955: how the Jonas Salk polio vaccine shaped regulation
The manufacturing scare reshaped the spine of vaccine oversight. Regulators revised release criteria, tightened documentation, and normalized the idea that a program can pause to protect the public and then resume stronger than before. The lesson generalized beyond polio: assume nothing, verify everything. Through that lens, the Jonas Salk polio vaccine did more than curb a single disease—it taught a country how to supervise complex biologics without suffocating innovation.
In clinics, the change felt concrete. Nurses learned new checklists; plants adopted extra inactivation assays; statisticians strengthened monitoring plans for rare adverse events. Parents noticed, too—not the assays, but the candor. The ability to acknowledge error, explain it plainly, and show the fix in detail made the Jonas Salk polio vaccine a case study in how institutions earn second chances.
The broader vaccine ecosystem grew more disciplined. Independent boards became routine. Post-marketing surveillance gained sharper teeth. And the phrase “out of an abundance of caution” matured from a cliché into a governing principle. Far from undermining confidence, these changes deepened it; they showed that the safety net had layers and that the Jonas Salk polio vaccine sat inside a system designed to catch what individuals might miss.
Where the Jonas Salk polio vaccine sits in the eradication story
Global campaigns required choreography rather than rivalry. In places with intense transmission and limited infrastructure, an oral, live-attenuated option excelled at stopping spread. In settings where transmission ebbed and primary care was stable, the Jonas Salk polio vaccine provided a conservative risk profile well suited to long-term routines. Used together, the tools drove wild poliovirus to historic lows and kept it there.
As eradication neared, many countries emphasized inactivated shots for routine protection, while still deploying targeted tools in outbreaks. The balance varied by epidemiology, but the logic stayed steady: pick the instrument that matches the moment. In that pragmatic frame, the Jonas Salk polio vaccine continued its quiet work—protecting nerves one child at a time while the world’s map of paralysis kept shrinking.
The final miles of eradication are logistical more than conceptual. They demand relentless routine coverage, speed in outbreak response, and surveillance sturdy enough to detect the rarest signals. Those tasks may be unglamorous, but they are what make victories durable. In the background, the Jonas Salk polio vaccine remains a dependable constant, the dependable anchor of schedules that parents and clinicians can set their watches by.
Field trials as community theater (without the spotlight)
The great field studies doubled as a kind of civic theater in which everyone had a role. Principals sent forms home in backpacks; school nurses arranged lines in gymnasiums; civic groups kept children calm with juice and crackers; statisticians balanced power against bias in quiet rooms. Randomization and blinding weren’t abstractions; they were practical tools for honesty. The separation between developers and evaluators wasn’t a bureaucratic hoop; it was the engine of credibility.
In the aftermath, the “Polio Pioneers” weren’t just subjects; they were acknowledged as participants. Communities received updates, not just outcomes. And when a frightening manufacturing problem hit the news, those same communities watched authorities pause, explain, and repair. That choreography—action, transparency, correction—became a public health muscle memory drawn upon in later decades whenever a new vaccine reached clinic doors.
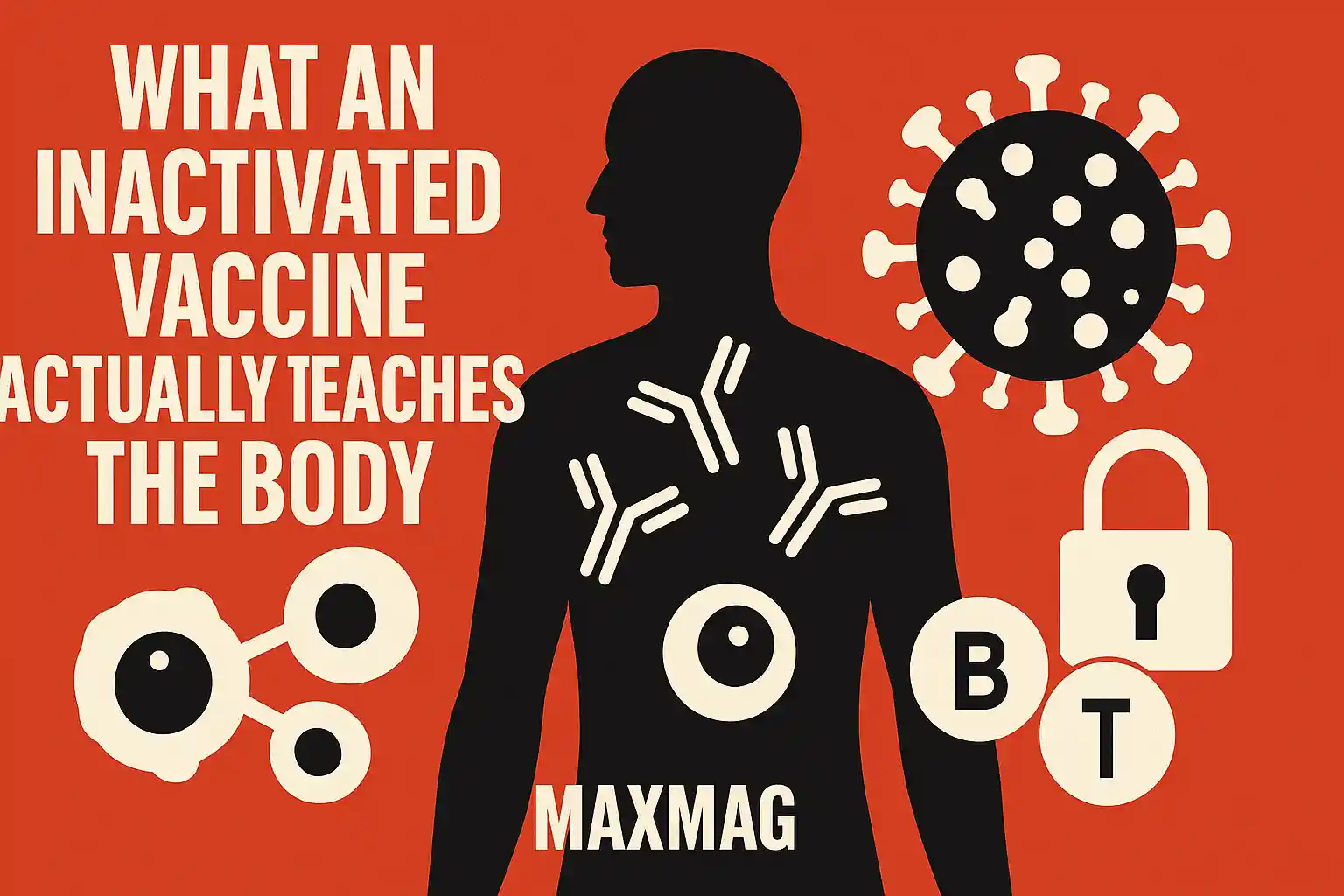
What an inactivated vaccine actually teaches the body
Imagine showing the immune system a detailed photograph of an intruder’s face. That’s the essence of inactivated vaccine design: a faithful likeness without the danger of a living threat. B cells learn to make neutralizing antibodies; T cells learn recognition patterns; memory cells file the lesson for later. The trick is preserving the critical three-dimensional shapes during inactivation so that the “photograph” is crisp, not blurred. This chemistry has practical consequences in policy, too—because the virus is dead, it cannot replicate or shed, which simplifies certain risk calculations in communities where the disease is already rare.
There’s also an industrial moral. Innovation happens at benches; reliability is proven on assembly lines. Lot after lot must be identically inactivated, identically potent, identically pure. That demand turned biologics manufacturing into a public safety profession, one in which technicians, engineers, and quality specialists became as vital to childhood protection as the scientists whose names fill textbooks.
Communication that didn’t overpromise
Public-facing materials from the era were strikingly plain. Instead of glossy pitches, pamphlets relied on unadorned sentences: what polio is, what the shot does, when a child should receive it, which mild reactions might follow, and when to call a doctor. Pediatric societies aligned calendars and contraindications with local departments. Radio spots and church bulletins traded hype for clarity. The result was uptake that felt less like a campaign and more like a commonsense routine.
In that routine lived the real miracle: the conversion of dread into habit. The encounter moved from front-page news to refrigerator calendars; from mass fear to individual planning. That ordinariness is the true monument to the work of Salk and his colleagues. Statues and institutes matter; the small ritual of a shot matters more.
The human being at the center
Jonas Salk’s reputation often hangs on one quotation—“Could you patent the sun?”—but the ethic behind it ran deeper than a headline. He understood that in democracies, legitimacy is partly moral. It’s about what something is for. The vaccine was for children, for summers reclaimed, for wheelchairs that never had to be bought. Choosing not to patent was a gesture as much as a legal decision; it told the country that the point was protection, not profit. Whether or not any patent would have been viable is secondary to the message it sent: this was a public good.
He also believed in understatement. When early data looked promising, he avoided superlatives. He favored measured sentences that could survive a tough question. That restraint, echoed by the physicians and nurses who implemented the program, became a form of respect. It said: we will not sell you magic; we will show you results.
Policy lessons still worth using
First, separate enthusiasm from evaluation. Great inventors are often poor judges of their own work; independent analysis protects both the public and the inventor.
Second, practice transparency as technique. Plain documentation of benefits and risks is not PR; it is infrastructure.
Third, rehearse logistics before the big day. Approval without distribution is an empty victory.
Fourth, treat course corrections as normal. Systems that can pause and repair are safer than systems that pretend nothing ever breaks.
Fifth, speak to people like adults. Respectful language travels further than slogans.
The day Ann Arbor rang the bells
When the trial results were announced, some towns rang church bells; some families cried in kitchens; some schoolkids cheered. The mood was not triumphalist so much as relieved. Relief quickly turned to logistics: allocating lots, scheduling clinics, training vaccinators, and setting up post-marketing surveillance. Headlines fade; calendars remain. In that pivot from celebration to scheduling you can see the whole philosophy: make prevention ordinary, and crises will spend more time in archives than in everyday life.
A legacy you can touch
Walk into a pediatric clinic today and you will see the inheritance everywhere: in the schedules printed on clipboards, in the calm way a nurse coaches a nervous child, in the paperwork that explains risks without drama. That ordinariness is the durable victory. The inheritance of mid-century work is not only lower paralysis rates. It is a model for how a country can reason together about risk, share burdens, and move forward with empathy.









