Imagine touching a small device to damaged skin and watching the body heal itself—no invasive surgery, no complicated drugs, no foreign substances. That’s the remarkable promise behind a breakthrough in biotechnology: a cell reprogramming chip capable of turning ordinary skin cells into the building blocks of new tissues. Developed by researchers at The Ohio State University Wexner Medical Center, this tiny chip uses cutting-edge nanotechnology to spark regeneration in limbs, blood vessels, and even brain cells.
For decades, scientists have dreamed of tissue regeneration using a patient’s own cells. Now, with the help of this innovative device, that dream may soon become a clinical reality.
A Breakthrough in Cell Reprogramming Technology
The concept of cell reprogramming isn’t new. Scientists have long explored ways to convert one type of cell into another. Traditional methods typically involve inserting foreign genetic material into cells using viruses as delivery systems. While effective in some contexts, these methods pose serious risks: viruses can infect unintended cells, trigger harmful immune responses, and even cause tumors.
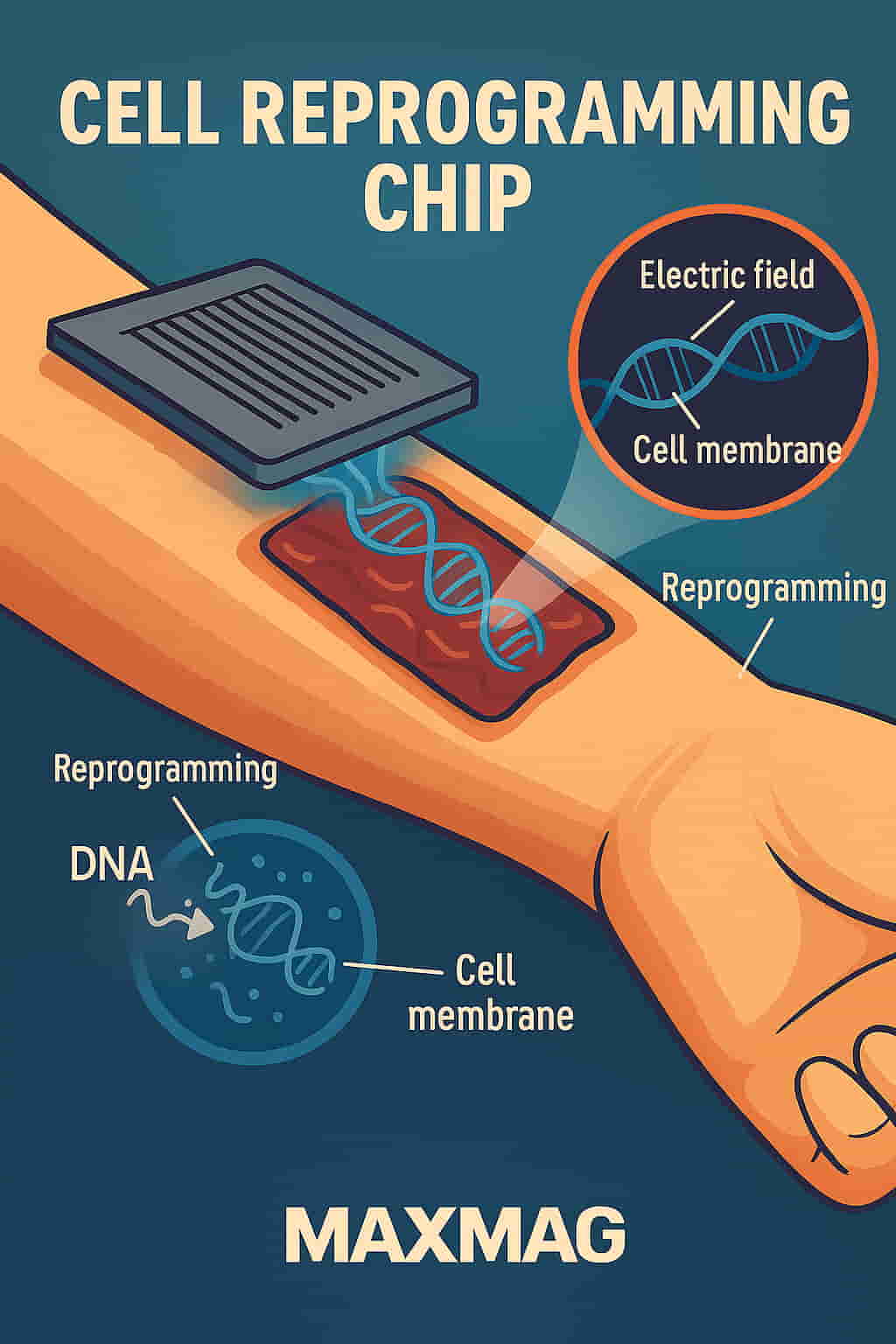
The cell reprogramming chip presents a safer, more precise alternative. Instead of relying on viral vectors, the chip uses a series of microscopic channels to direct a controlled electrical pulse to a cell’s surface. This electrical field opens a tiny, temporary pore in the cell membrane—just enough to allow reprogramming material to pass through without damaging the cell.
James Lee, a chemical and biomolecular engineer involved in the project, explained that the process is like creating a microscopic doorway. “We’re not disrupting the entire cell,” he said. “We’re introducing instructions exactly where they’re needed, in the right dosage and location.”
According to the National Institutes of Health, non-viral gene delivery methods like this one are considered a major leap forward in the field of gene therapy.
From Mouse Trials to Medical Milestones
In their laboratory experiments, the research team tested the chip on mice with severely injured legs. These injuries were caused by blocked arteries that cut off blood flow—mimicking a condition that can lead to tissue death in humans. The chip was applied directly to the surface of the skin. With one brief touch, genetic instructions were delivered to the skin cells, reprogramming them into endothelial cells—the essential cells that form blood vessels.
What happened next was nothing short of astonishing. New blood vessels began forming within days. Blood flow was restored to the damaged tissue. After just three weeks, the injured legs had completely healed.
And all it took was one application.
Chandan Sen, the lead scientist on the project, noted that the success of the treatment was due to the rapid transformation of skin cells into a functional vascular network. The mice not only regained function but showed no signs of immune rejection or adverse effects.
This is a stark contrast to older approaches, where only a small percentage of cells could be successfully reprogrammed—and many were damaged or killed in the process.
Healing Beyond the Surface
Perhaps even more compelling is what the scientists discovered after the initial cell transformation. The reprogrammed cells began releasing extracellular vesicles—tiny particles that carry proteins, RNA, and other materials used to signal nearby cells. These vesicles traveled deeper into surrounding tissues, spreading the healing message.
The team extracted these vesicles and injected them into other mice with similar injuries. Incredibly, the healing effect was the same. Even without using the chip directly, the tissue regeneration process was triggered.
This finding points to the possibility of developing injectable regenerative therapies using vesicles derived from reprogrammed cells—offering a potential non-invasive alternative for patients who might not be ideal candidates for direct chip application.
Harvard Medical School has also highlighted the medical significance of extracellular vesicles, especially in targeted therapy and regeneration.
Brain Regeneration After Stroke
The chip’s potential doesn’t end with blood vessels. The researchers also experimented with reprogramming skin cells into neuronal cells. In a separate study, they implanted these newly transformed neurons into the brains of mice that had suffered strokes. The result: measurable improvement in the animals’ cognitive functions.
Dr. Benedikt Berninger, a neurobiologist from Johannes Gutenberg University in Germany, commented that the ability to influence brain recovery using locally derived cells was “a profound step forward” in neuroregenerative science. Though he was not involved in the study, he acknowledged the proof-of-concept as groundbreaking.
For stroke victims and patients suffering from neurodegenerative disorders, this chip may eventually offer a personalized path to recovery—one that doesn’t rely on donor cells or synthetic materials.
How It Works: Tissue Nanotransfection
This new approach is known as tissue nanotransfection. The name may sound complex, but the process is elegantly simple. The chip is placed directly onto the skin. A small electrical pulse is administered, and genetic cargo is transferred into the cells via minuscule pores created in their membranes.
Unlike older methods like electroporation, which bombard entire tissues with electric fields and often cause cell death, tissue nanotransfection works at the single-cell level with pinpoint accuracy.
The chip’s architecture contains microfluidic channels, which allow researchers to control exactly which cells are targeted and how much material is delivered. This high level of control is essential not only for safety but for achieving reproducible results—something that older, bulk-delivery methods struggle with.
You can learn more about microfluidic technology and its medical applications through MIT’s Research Laboratory of Electronics.
Clinical Potential and the Road Ahead
While all of the current data comes from preclinical mouse trials, the research team believes the cell reprogramming chip is safe enough for eventual human testing. Discussions with the U.S. Food and Drug Administration (FDA) are underway, and early-stage planning for clinical trials has begun.
Unlike some cutting-edge therapies that require expensive lab-grown stem cells or complex surgical procedures, this chip could be deployed in a standard clinical setting. A single outpatient visit, a five-minute application, and the body could begin healing itself from within.
The implications are massive: chronic wounds, ischemic limbs, nerve damage, and even early-stage Alzheimer’s could all be future targets for this technology.
The Cleveland Clinic has already identified regenerative medicine as one of the most promising fields of the 21st century. Technologies like this chip are poised to lead that charge.
Why the Cell Reprogramming Chip Stands Out
Compared to conventional tissue repair and cell therapy options, the cell reprogramming chip offers:
-
Minimally invasive treatment with no needles or incisions
-
On-site cell transformation, using the patient’s own tissue
-
Avoidance of viral vectors, reducing risk of infection or mutation
-
Scalable design, potentially usable in rural clinics or emergency settings
-
Low cost and rapid application, making it ideal for mass deployment
What makes this innovation even more exciting is its versatility. The same chip could potentially be programmed with different genetic recipes to produce a wide variety of cell types—nerve, muscle, cartilage, or even pancreatic cells.
A Glimpse Into the Future
The vision is clear: one day, this chip could be part of every trauma room, burn unit, or stroke rehabilitation center. Rather than replacing damaged tissues with prosthetics or relying on donor organs, doctors could trigger in-body regeneration using the patient’s own skin as the source.
While more testing is needed, the science is moving fast—and with such promising results in animals, clinical human trials may not be far behind.
If successful, this could mark the beginning of a new era where cell reprogramming chips enable us not just to treat disease, but to truly regrow what’s been lost.
Frequently Asked Questions (FAQ)
🧬 What is a cell reprogramming chip?
It’s a nano-scale device that uses a mild electrical signal to deliver genetic instructions into skin cells, converting them into other functional cell types like neurons or endothelial cells.
🧠 Can it repair brain injuries?
In preclinical trials, researchers successfully reprogrammed skin cells into neurons and implanted them in the brains of mice that had suffered strokes, resulting in improved mental function.
🩺 Is it safe for humans?
The technique avoids using viruses and doesn’t involve surgery, making it significantly safer than many traditional gene therapies. Human trials are expected in the near future.
🧪 How does it compare to stem cell therapy?
Unlike stem cell therapy, which involves external cell cultivation and reinjection, the chip reprograms cells directly inside the body, speeding up treatment and minimizing risk.
🚀 Where can I learn more?
Research is ongoing at The Ohio State University Wexner Medical Center, a leader in regenerative and nano-medicine.