The idea of a brain‑computer interface via blood vessels is ushering in a new era of neurotechnology. Imagine controlling a computer or prosthetic limb not with invasive brain surgery but through a network of electrodes threaded gently into blood vessels. This approach, which drastically reduces medical risks, could transform the way we interact with machines and treat neurological disorders. In this article, we dive into how this revolutionary concept works, who’s developing it, and what it means for the future of medicine and human augmentation.
What Is a Brain‑Computer Interface via Blood Vessels?
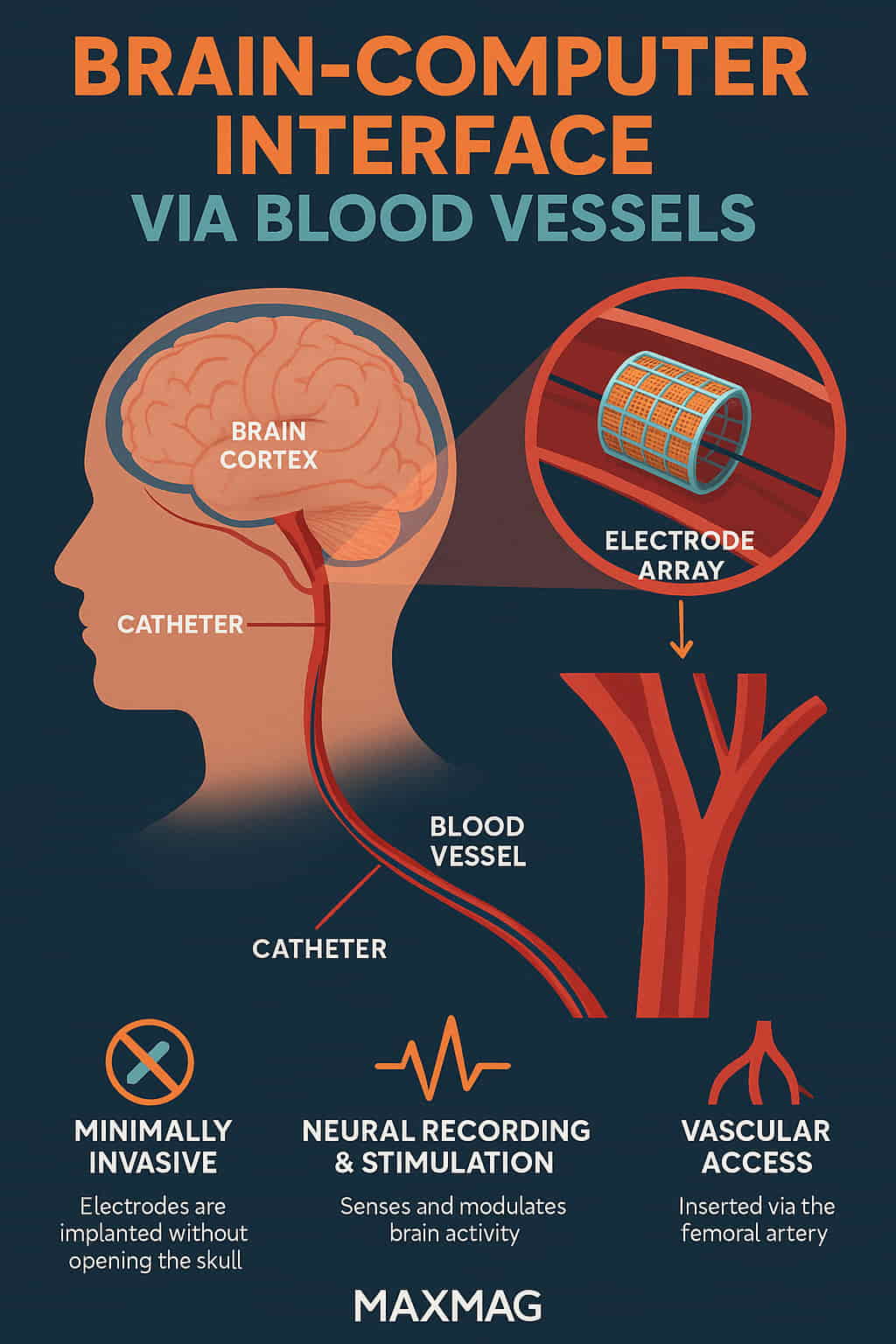
A brain‑computer interface via blood vessels (BCI-BV) is a type of neurotechnology that allows electrodes to be placed within the brain’s blood vessels to record neural activity or stimulate brain function. Instead of opening the skull, doctors guide a catheter through the vascular system to deploy a stent-like device known as a “stentrode.” This device contains tiny electrodes that sit next to the brain’s cortex and can either read signals or deliver stimulation.
This technique draws from endovascular surgery, a well-established field used in treating strokes and aneurysms. According to Johns Hopkins Medicine, catheter-based interventions offer minimally invasive solutions for complex neurological problems—now extended into brain-machine interfacing.
Why Is the Brain‑Computer Interface via Blood Vessels Important?
Minimally Invasive and Safer
Traditional brain-computer interfaces often require a craniotomy, which comes with the risk of infection, bleeding, and prolonged recovery. In contrast, a brain‑computer interface via blood vessels involves a procedure similar to an angiogram. Patients often return home within days. According to the Cleveland Clinic, endovascular methods generally have faster recovery times and fewer complications than open surgery.
Access to Previously Inaccessible Brain Regions
Blood vessels span the entire brain. This allows doctors to access and interface with brain regions previously unreachable through traditional surgery, enhancing treatment possibilities for conditions like epilepsy and Parkinson’s disease.
Long-Term Signal Stability
Vascular implants have been shown to cause less tissue damage than surface implants. That translates into more consistent neural signal collection over time, which is critical for patients with chronic disorders or degenerative diseases.
How It Works
-
Catheter Insertion: The process begins with a small incision, typically in the groin, where a catheter is inserted into a vein or artery.
-
Guided Navigation: Surgeons navigate the catheter through the vascular system to the brain using real-time imaging.
-
Stentrode Deployment: Once in place, a stent-like device containing electrodes is deployed in a major blood vessel near the motor cortex.
-
Signal Collection: Electrodes detect neural activity through the blood vessel walls.
-
Data Transmission: Signals are transmitted wirelessly to a device, which decodes them into commands for computers, wheelchairs, or robotic limbs.
-
Feedback Loop: In therapeutic scenarios, the system can also send electrical impulses back to the brain to modify neural activity.
This design has been successfully demonstrated by Synchron, a U.S.-based neurotech company that received breakthrough designation from the FDA for its endovascular brain interface.
Advantages of the Brain‑Computer Interface via Blood Vessels
-
Low Invasiveness: No need for open-skull surgery or removal of skull portions.
-
Quicker Recovery: Many patients are discharged within 48 hours.
-
Reduced Risk of Infection: No direct contact with brain tissue.
-
Expanded Access: Electrode placement in deep and sensitive areas.
-
Scalability: Potential to place multiple devices in different vascular areas for broader neural interfacing.
This approach is already being used to assist patients with ALS in controlling digital devices, as featured in a recent study covered by MIT Technology Review.
Challenges and Concerns
Blood Vessel Health
One of the primary concerns is how the implanted electrodes affect blood flow and whether they could cause clotting. Research is ongoing, and stentrodes are being coated with anti-clotting agents and biocompatible materials.
Data Bandwidth
Current vascular electrodes offer lower resolution compared to those directly placed on the brain. However, machine learning algorithms are helping to bridge the gap by enhancing decoding capabilities.
Longevity of Implants
Though promising, long-term durability and safety still require years of clinical trials. According to the National Institutes of Health (NIH), brain-interface devices must undergo rigorous testing to ensure long-term biocompatibility and functionality.
Ethical Considerations
As BCIs become more advanced, questions about privacy, consent, and cognitive liberty grow. Who owns your neural data? What protections exist against misuse? These are not purely technological but deeply philosophical issues.
Latest Developments in Brain‑Computer Interface via Blood Vessels
Synchron’s system—the first to be permanently implanted in a human via blood vessels—continues to break ground. Their BCI was used by patients with paralysis to send emails and texts using only their thoughts. According to Nature, this method allows stable communication for months with minimal signal degradation.
The U.S. Department of Veterans Affairs has partnered with Synchron to explore applications in spinal cord injury rehabilitation. The company is currently undergoing large-scale trials in the U.S. under the FDA’s Breakthrough Devices Program.
How Does It Compare to Traditional BCIs?
| Feature | Traditional BCIs | Brain-Computer Interface via Blood Vessels |
|---|---|---|
| Invasiveness | High (craniotomy required) | Low (catheter-based) |
| Recovery Time | Weeks | Days |
| Infection Risk | Moderate to High | Very Low |
| Signal Stability | Moderate | High |
| Potential Applications | Clinical & research-focused | Broadening to consumer & medical use |
The Future of Brain-Machine Communication
As technology improves, we may witness BCIs being used not just for medical needs but for enhancing human capabilities—such as cognitive enhancement, memory recall, or immersive gaming. Elon Musk’s Neuralink is also pursuing BCIs, albeit via a different surgical path, showing broad industry momentum toward brain-based control systems.
For now, the brain‑computer interface via blood vessels stands out for its blend of safety, practicality, and early clinical success. With continued research and funding, this technology could soon be as common as a pacemaker or insulin pump.
FAQ
Q1: How safe is the brain‑computer interface via blood vessels?
Studies show it’s significantly safer than open-brain surgery. So far, there have been no major complications in human trials. Devices like the stentrode are designed to minimize clotting and inflammation.
Q2: Who is a candidate for this procedure?
Patients with paralysis, ALS, or locked-in syndrome are current candidates. Broader uses are being explored, including in stroke and Parkinson’s treatment.
Q3: What kind of tasks can patients perform with this technology?
Typing emails, controlling smart home devices, browsing the internet—all through thought alone.
Q4: Is it permanent?
Yes. The stentrode is designed for long-term use, although more data is needed on durability over multiple years.
Q5: What are the long-term goals of this tech?
Beyond helping disabled individuals, researchers envision using it for treating depression, enhancing cognition, and even enabling thought-based communication between humans.