The story told in any serious Emmanuelle Charpentier biography begins far from the headlines about gene-editing revolutions and Nobel Prizes. It starts in a quiet commuter town just south of Paris, with a curious girl who loved microscopes, mysteries and the hidden life of microbes. Decades later, that same curiosity would help give the world CRISPR-Cas9, a set of molecular scissors that let scientists edit DNA with unprecedented precision, and would turn Emmanuelle Charpentier into one of the most influential figures in modern genetics. The Emmanuelle Charpentier biography is not just a tale of scientific brilliance, but also of persistence, doubt, rivalry and the ethical questions that arrive when humans gain the power to rewrite the code of life.
Emmanuelle Charpentier at a glance
- Who: Emmanuelle Charpentier is a French microbiologist, geneticist and biochemist, best known as a CRISPR pioneer.
- Field and era: Late 20th and early 21st century molecular biology, infection biology and genome editing.
- Headline contribution: Co-discovery of the CRISPR-Cas9 gene-editing tool, which allows targeted changes to DNA in plants, animals and humans.
- Why she matters today: Her work underpins experimental therapies for genetic diseases, fuels debates over gene-edited babies and shapes the future of biotechnology and medicine.
Early Life and Education in the Emmanuelle Charpentier biography
A Paris childhood that shaped the Emmanuelle Charpentier biography
Emmanuelle Charpentier was born in December 1968 in Juvisy-sur-Orge, a modest town just outside Paris. She grew up in an era when biology was stepping out of the shadow of classical medicine and beginning to speak the language of genes and molecules. At school she was the kind of pupil teachers remember: quiet, meticulous, but with a fierce determination to understand how things worked beneath the surface. Friends recall that she was drawn more to laboratories than to loud crowds, more to chemistry sets than to typical teenage distractions. In retrospect, those early preferences read like foreshadowing in the Emmanuelle Charpentier biography, but at the time they were simply an extension of a child’s curiosity.
Her parents were not scientists, but they encouraged her to pursue demanding studies and to leave space for ambition. The young Charpentier devoured popular science books and was fascinated by stories of microbes causing – and sometimes curing – disease. The idea that tiny organisms could tip the balance between health and catastrophe gave her a narrative to follow: perhaps she, too, could learn to read and rewrite those invisible dramas.
From student in Paris to microbiology pioneer
Charpentier studied biochemistry and microbiology at the Pierre and Marie Curie University in Paris, a training ground for many European scientists. There she encountered the discipline that would define her career: molecular biology, the study of life at the level of DNA, RNA and proteins. Her doctoral work focused on antibiotic resistance in Listeria, a bacterium that can cause serious infections. While the details were technical, the underlying story was simple enough: bacteria are constantly evolving ways to evade our drugs, forcing scientists to keep up in an endless arms race.
This early work sharpened her skills in genetics, microbiology and experimental design. It also gave her a lasting respect for pathogens as both enemies and teachers. The bacteria she studied were dangerous to patients, but they were also revealing clues about how genetic systems adapt and fight back. Without knowing it, she was already circling themes that would later dominate the Emmanuelle Charpentier biography: defense systems, genetic memory and the possibility of turning a microbe’s weapons into human tools.
By the time she completed her PhD, Charpentier had already decided that her career would not be confined to one country. She wanted to see how biology was practiced in different cultures and laboratories, and she was willing to start again in unfamiliar cities to do it. That restlessness would take her to New York, Vienna, Umeå and Berlin, and would give the Emmanuelle Charpentier biography an unusually international flavour.
Emmanuelle Charpentier biography and the birth of CRISPR
Crossing the Atlantic: New York, new mentors, new questions
In the late 1990s, Charpentier moved to the United States for postdoctoral work at the Rockefeller University in New York. It was a classic rite of passage for a young scientist: long days at the bench, new techniques to master, and the unsettling excitement of feeling both lost and in exactly the right place. In New York she worked on pathogenic bacteria and how they interact with their hosts, learning to see microbes as players in dynamic ecosystems rather than simple villains.
These years in American labs added another layer to the Emmanuelle Charpentier biography: exposure to a culture of bold experimentation and collaboration. She learned how quickly ideas could move when physicists, clinicians and biologists shared corridors and coffee breaks. But she also discovered the precarious nature of scientific careers – grants that ran out, projects that failed, visas that needed renewal. The romantic notion of the lone genius gave way to a more realistic picture: science as a team sport, often played under chronic uncertainty.
The Scandinavian detour that changed modern genetics
In the 2000s, Charpentier moved again, this time to Umeå University in northern Sweden. It was not the obvious place one would expect a breakthrough in global gene-editing technology to emerge from. Yet the quiet, snow-bound city offered something invaluable: time and space to think, and a supportive environment for risky basic research.
In Umeå, she focused on Streptococcus pyogenes, a bacterium better known for causing strep throat. At the time, a handful of researchers were beginning to pay attention to strange repeating sequences in bacterial genomes, known by the acronym CRISPR. These snippets, it turned out, were part of a primitive immune system that allowed bacteria to recognise and cut up invading viruses. Charpentier’s group homed in on a particular RNA molecule, tracrRNA, that seemed to be crucial to this process.
It was painstaking work, the sort of detailed, step-by-step investigation that rarely makes headlines but underpins every scientific revolution. By showing that tracrRNA was essential for processing CRISPR RNAs and guiding the bacteria’s molecular scissors, Charpentier helped reveal how this microbial immune system actually functioned. The CRISPR-Cas9 history would later be written in terms of patents and prizes, but at its core lay this careful dissection of genetic machinery in a far-flung Scandinavian lab.
The Puerto Rico meeting that redirected the Emmanuelle Charpentier biography
The turning point in the Emmanuelle Charpentier biography often appears in a single scene: a scientific conference in San Juan, Puerto Rico, in 2011. There, Charpentier met Jennifer Doudna, an American biochemist from the University of California, Berkeley. Doudna was an expert in the structure of RNA molecules; Charpentier brought deep knowledge of CRISPR in bacteria. Over coffee and conversations, the two realised that together they might be able to turn a bacterial curiosity into a programmable tool.
Their collaboration, spanning continents and time zones, led to a landmark 2012 paper describing how Cas9, guided by RNA, could be directed to cut DNA at chosen sites. In plain terms, they had demonstrated a relatively simple way to edit genes – a method that could, at least in principle, be used across many species. This was the moment when CRISPR leapt from being a niche topic in microbiology to becoming the engine of a genome editing revolution. From that point on, every serious Emmanuelle Charpentier biography would be inextricably tied to debates about what humans should, and should not, do with such power.
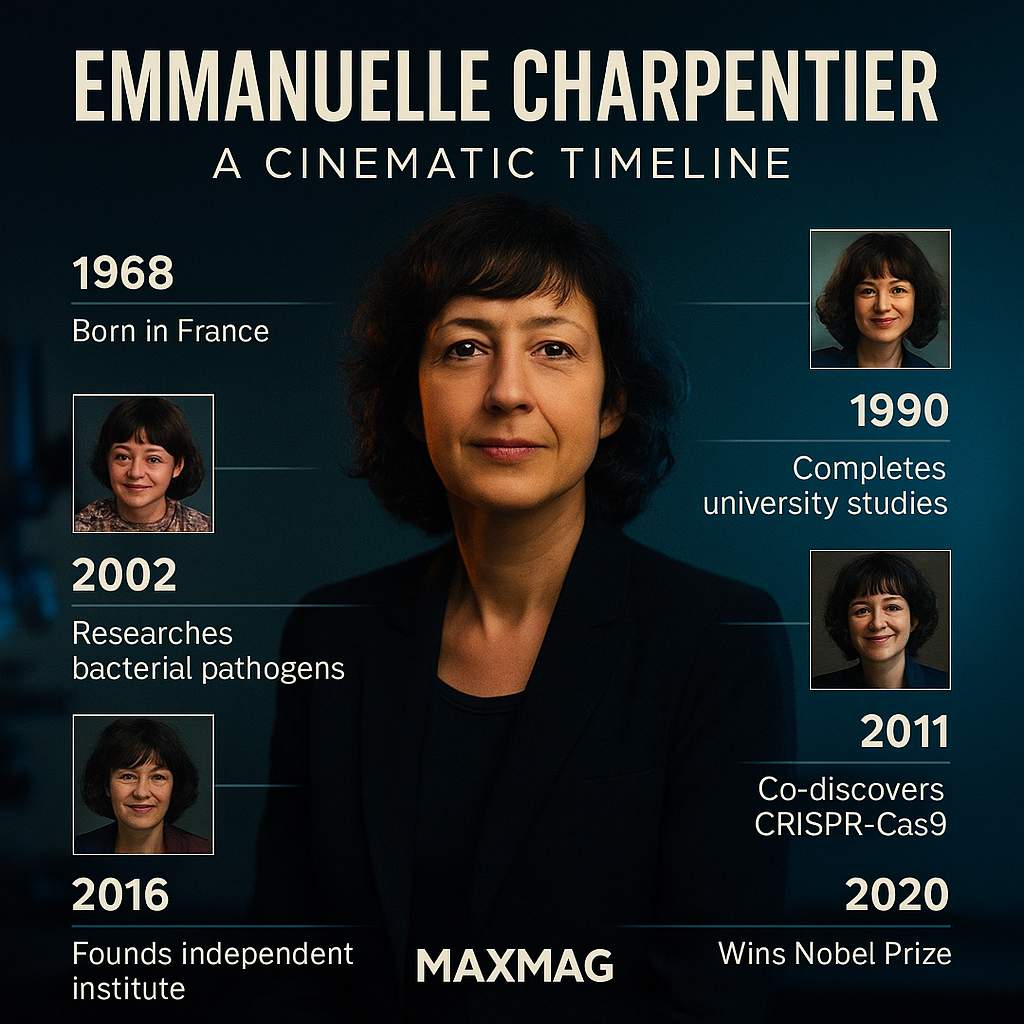
Key Works and Major Contributions of Emmanuelle Charpentier
Decoding a bacterial immune system
Charpentier’s first major contribution was to show how bacteria record the genetic signatures of viral invaders and use them to defend themselves. In the CRISPR system, bacteria store short fragments of viral DNA as a kind of molecular mugshot gallery. When the same virus reappears, RNA copies of those fragments guide the Cas9 enzyme to slice up the intruder’s DNA. By clarifying the roles of tracrRNA and CRISPR RNAs in this process, Charpentier helped turn a cryptic acronym into a coherent story.
For non-specialists, one analogy helps: imagine a high-tech security system that photographs every burglar and files those images in a database. The next time someone with that face approaches, an alarm triggers and a robotic arm locks the door. In Charpentier’s hands, CRISPR-Cas9 became that security system, but one that could be reprogrammed to recognise whatever DNA sequence scientists chose.
From basic science to gene-editing technology
The 2012 paper with Doudna did more than describe an elegant mechanism. It provided a recipe that other researchers could follow to edit genes in their own systems. Within months, laboratories around the world were using CRISPR-Cas9 to knock out genes in mice, tweak crops, and probe the genetic roots of human diseases. The barrier to entry was much lower than for previous gene-editing techniques, and a new wave of molecular biology research followed.
Charpentier did not stop at the conceptual breakthrough. She co-founded CRISPR Therapeutics and ERS Genomics, commercial ventures aimed at translating CRISPR into real therapies. It was a risky move into the world of biotech entrepreneurship, but a logical extension of her view that fundamental discoveries should eventually benefit patients, not just earn citations.
A Nobel Prize and global recognition
In 2020, Emmanuelle Charpentier and Jennifer Doudna were awarded the Nobel Prize in Chemistry for the development of CRISPR-Cas9. It was the first time a science Nobel had gone exclusively to two women, and the announcement was rightly hailed as a milestone for gender representation as well as for genetics. For Charpentier, the prize was both a personal honour and a validation of decades of work on bacterial systems many had once dismissed as obscure.
The Nobel committee’s popular explanation of their decision emphasised not only the power of CRISPR to reshape genetics, but also its potential for new cancer treatments and cures for inherited diseases. Outside the lab, the award turned Charpentier into a public figure, a symbol of both scientific progress and the ethical dilemmas it raises.
Methods, Collaborations and Working Style
Austere labs, meticulous notebooks
Colleagues describe Charpentier’s working style as methodical and demanding, with a strong emphasis on careful controls and reproducibility. She is not the stereotype of the flamboyant scientific showman; instead, she tends to let the data speak first. Former students recall that she expected experiments to be logged in detail and results to be questioned relentlessly, even when they seemed to confirm her hypotheses.
This rigor is a thread that runs through the Emmanuelle Charpentier biography. The leap from bacterial defense system to programmable gene-editing tool was only possible because every intermediate step had been tested, retested and challenged. In an era when flashy results can bring rapid fame, her insistence on solid foundations is part of what sets her apart as a CRISPR pioneer.
Collaboration across borders
Charpentier’s career illustrates how modern science depends on international networks. She has worked in France, the United States, Austria, Sweden and Germany, often moving at points when it would have been easier to stay put. Each move brought new techniques, perspectives and collaborators, and helped shape a more global vision of microbiology and genetics.
Her partnership with Doudna is the most famous of these collaborations, but not the only important one. Charpentier has repeatedly sought out expertise she lacked – in structural biology, clinical research, or biotechnology – and woven it into her projects. In that sense, the Emmanuelle Charpentier biography is also a story about the value of humility in science: knowing when to lead, and when to let others’ skills take centre stage.
Founding a new research unit
After her CRISPR breakthrough, Charpentier became the founding director of the Max Planck Unit for the Science of Pathogens in Berlin. The institute is deliberately small and focused, aiming to give researchers the freedom to pursue ambitious questions about infection and immunity without constant grant-writing pressure. It reflects her belief that transformative discoveries often emerge from long, uninterrupted engagement with a difficult problem.
A detailed University of Florida genetics symposium profile highlighted how Charpentier uses such leadership roles to advocate for basic research, arguing that today’s curiosity-driven experiments are tomorrow’s medical breakthroughs (University of Florida genetics symposium profile on Emmanuelle Charpentier). It is a reminder that behind the headlines about Nobel Prizes and gene-editing technology lies a sustained commitment to fundamental questions about pathogens and hosts.
In short, her methods and leadership style show a scientist who values patience over spectacle, collaboration over ego and depth over breadth.

Controversies, Criticism and Misconceptions
Who owns CRISPR?
No modern Emmanuelle Charpentier biography can avoid the tangled story of CRISPR patents. After Charpentier and Doudna published their landmark work, other groups – most prominently the Broad Institute in Cambridge, Massachusetts – filed patents on using CRISPR in certain types of cells. A web of lawsuits and appeals followed, raising difficult questions about how to reward scientific discovery without stifling innovation.
In recent years, U.S. courts and patent boards have revisited key decisions about who first conceived of CRISPR’s use in complex, eukaryotic cells. The legal arguments are dense, but the human story beneath them is familiar: competing teams, overlapping ideas and a system that often struggles to capture the collaborative reality of science. For Charpentier, the disputes have meant repeated returns to the public eye, not as a microbiology pioneer but as a central figure in a multibillion-dollar intellectual property battle.
Myths about a lone inventor
Popular accounts sometimes flatten CRISPR into a story of one or two heroic figures, erasing the contributions of earlier scientists who identified CRISPR sequences or probed their function. Charpentier herself has acknowledged that the field built on the work of many microbiologists, bioinformaticians and geneticists. Reducing the Emmanuelle Charpentier biography to a tale of solitary genius obscures the layered, cumulative nature of scientific progress.
Another misconception is that CRISPR-Cas9 was born fully formed as a medical tool. In reality, it emerged from curiosity-driven research into how bacteria survive viral attacks – research that had no obvious commercial payoff when it began. That history matters, especially when policymakers debate how to fund science: it shows that transformative technologies can spring from questions that seemed, at first glance, esoteric.
Taken together, the controversies and myths around CRISPR serve as a cautionary chapter in the Emmanuelle Charpentier biography, reminding us how easily public narratives can distort the messy truth of discovery.
Impact on Genetics and on Wider Society
Transforming the everyday work of laboratories
Before CRISPR-Cas9, editing genes was possible but painstaking, expensive and often unreliable. With Charpentier’s work, gene editing became faster, cheaper and more accessible. In many labs today, designing a CRISPR experiment is as routine as ordering primers or culturing cells. This shift has accelerated research in fields from plant breeding to neuroscience, and has allowed modern genetics to ask questions that would have been unthinkable a decade ago.
The impact of this change is hard to overstate. When historians of science look back on early 21st-century biology, the arrival of CRISPR will rank alongside the discovery of DNA’s structure or the sequencing of the human genome. In that narrative, Emmanuelle Charpentier is one of the key architects of a new era.
Ethical debates and public anxiety
Alongside excitement, CRISPR has generated deep unease. If scientists can edit the genes of embryos, for example, should they be allowed to erase certain inherited diseases before a child is born? What about altering crops or wild species in ways that could spread through ecosystems? These questions go far beyond the walls of Charpentier’s laboratory and into the realms of law, philosophy and public opinion.
A widely read Washington Post piece on the Nobel announcement captured both the promise and the anxiety surrounding CRISPR, describing how the technology might cure inherited diseases while also raising fears of designer babies and new forms of inequality (Washington Post coverage of the 2020 Nobel Prize in Chemistry). In interviews, Charpentier has consistently urged caution and strong regulation, arguing that just because something is technically possible does not mean it is ethically acceptable.
These societal debates ensure that the Emmanuelle Charpentier biography will never be confined to specialist textbooks. Her work forces all of us to think about what should be changed in our genomes – and what should not.
Personal Beliefs, Character and Private Life
A reserved public figure
Compared with some scientific celebrities, Charpentier remains relatively private. She rarely shares details of her personal life and tends to steer interviews back to the science. Those who know her speak of a dry sense of humour, a dislike of grandstanding and a strong loyalty to her students and collaborators.
This reserve has led some to call her a “quiet revolutionary” – someone who changes the world not through constant media appearances, but through work done carefully, year after year. In the Emmanuelle Charpentier biography, this trait threads together childhood shyness, long hours in the lab and a measured approach to public debates about gene editing.
Views on responsibility and risk
Charpentier has repeatedly emphasised that CRISPR is both a powerful tool and a potential source of harm. She has called for robust ethical frameworks and international agreements to prevent reckless use, particularly in areas like germline editing, where changes could be inherited by future generations. For her, scientific freedom and responsibility are not opposites but obligations that must be held together.
These views place her among a generation of researchers who are unwilling to treat ethics as an afterthought. Instead, they see ethical reflection as part of the research process itself, shaping which experiments are imagined, designed and ultimately carried out.

Later Career and Ongoing Chapter of Emmanuelle Charpentier
Beyond the Nobel moment
Many scientists fear that a major prize will mark the end of their most creative period. In Charpentier’s case, the Nobel has been less a closing chapter than a new platform. At the Max Planck Unit for the Science of Pathogens, she continues to explore how microbes and hosts interact, and how those interactions might reveal new targets for therapy.
She also remains engaged with CRISPR Therapeutics and other efforts to turn gene-editing technology into real treatments. Clinical trials are under way for conditions such as sickle-cell disease and certain inherited blindnesses, and while Charpentier is not running those trials herself, her work underpins their design.
A continuing role in scientific debates
Patent disputes, international summits on genome editing and calls for moratoriums on certain applications all ensure that Charpentier stays involved in high-stakes conversations. The Emmanuelle Charpentier biography now includes appearances not just in scientific journals, but in court documents, ethics reports and policy recommendations.
As new versions of CRISPR – more precise, more flexible, potentially less error-prone – emerge, she is part of a broader community asking how these tools should be governed. Her perspective carries particular weight because she has seen the technology grow from a basic curiosity in bacterial genetics to a central instrument of biomedical innovation.
In effect, the later chapters of her life show a scientist learning to navigate the responsibilities that come with transforming not just a field, but society’s sense of what is possible.
The Lasting Legacy of the Emmanuelle Charpentier biography
A new template for scientific influence
The long view of the Emmanuelle Charpentier biography reveals more than a sequence of papers and prizes. It offers a template for how meticulous basic research can ripple outward into medicine, agriculture, ethics and law. CRISPR-Cas9 will continue to evolve, and future technologies may one day make it look primitive, but the conceptual leap it represents – from reading genomes to rewriting them – will always be associated with her name.
Her legacy also includes the example she sets for younger scientists: a French microbiologist who moved between countries and disciplines, who embraced collaboration, and who insisted that the excitement of discovery be matched by serious reflection on consequences. In a time when public trust in science can feel fragile, that combination of rigour and responsibility may be as important as any single experiment.
Ultimately, understanding the Emmanuelle Charpentier biography helps us understand something larger about our world. It shows how a question about how bacteria fight viruses can end up reshaping human medicine, agriculture and even the way we think about what it means to be human. As CRISPR-based therapies inch closer to routine clinical use, the story of Charpentier’s life and work will remain a touchstone in debates over how far – and how fast – we should go in editing ourselves.
Frequently Asked Questions about Emmanuelle Charpentier biography
Q1: Who is Emmanuelle Charpentier and why is she important in gene editing?
Q2: What is the connection between CRISPR-Cas9 and the Emmanuelle Charpentier biography?
Q3: Did Emmanuelle Charpentier make the CRISPR discovery alone?
Q4: What major awards feature in the Emmanuelle Charpentier biography?
Q5: Why are there patent disputes linked to Emmanuelle Charpentier and CRISPR?
Q6: How does the Emmanuelle Charpentier biography influence current debates on ethics and gene editing?